Biotech and pharmaceutical
Formulating effective biotechnology products can take years of effort, and careful selection of quality materials. You need to protect the customers who purchase and use your assays, components and kits, but you don’t want to reveal what makes your products better than those of your competitors.
SDScribe™ features convenient means to withhold ingredient names from the SDS, or entirely omit an ingredient that is non-hazardous.
Check a box next to the component to withhold the chemical name under US-OSHA regulations. Or enter a generic/alternate chemical name, as specified under Canadian (WHIMS) and European (EU-REACH) regulations (use of generic/alternate names may require agency approval).
SDScribe™ can generate an SDS in either the UN or EU-ECHA format from the same data. There are also separate, paired fields to enter the same information in a non-English language. With the optional non-English section titles, the program can generate SDSs in two languages from the same SDS record. These features make it easier for you to distribute to customers worldwide.
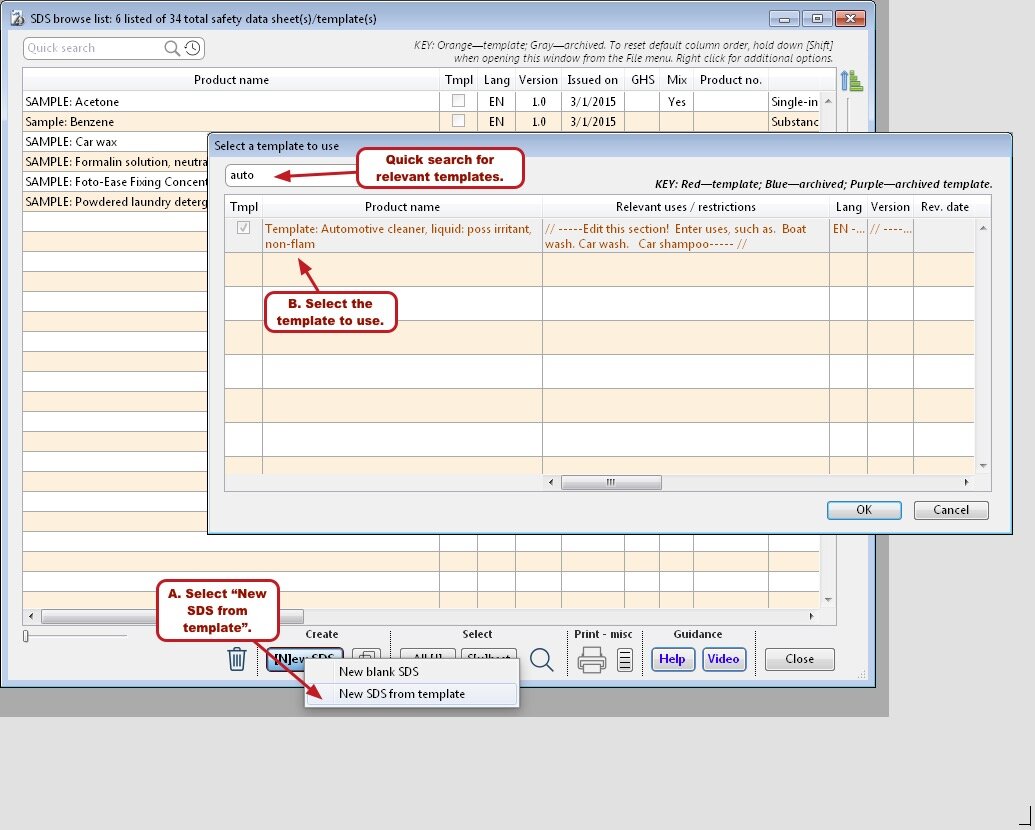
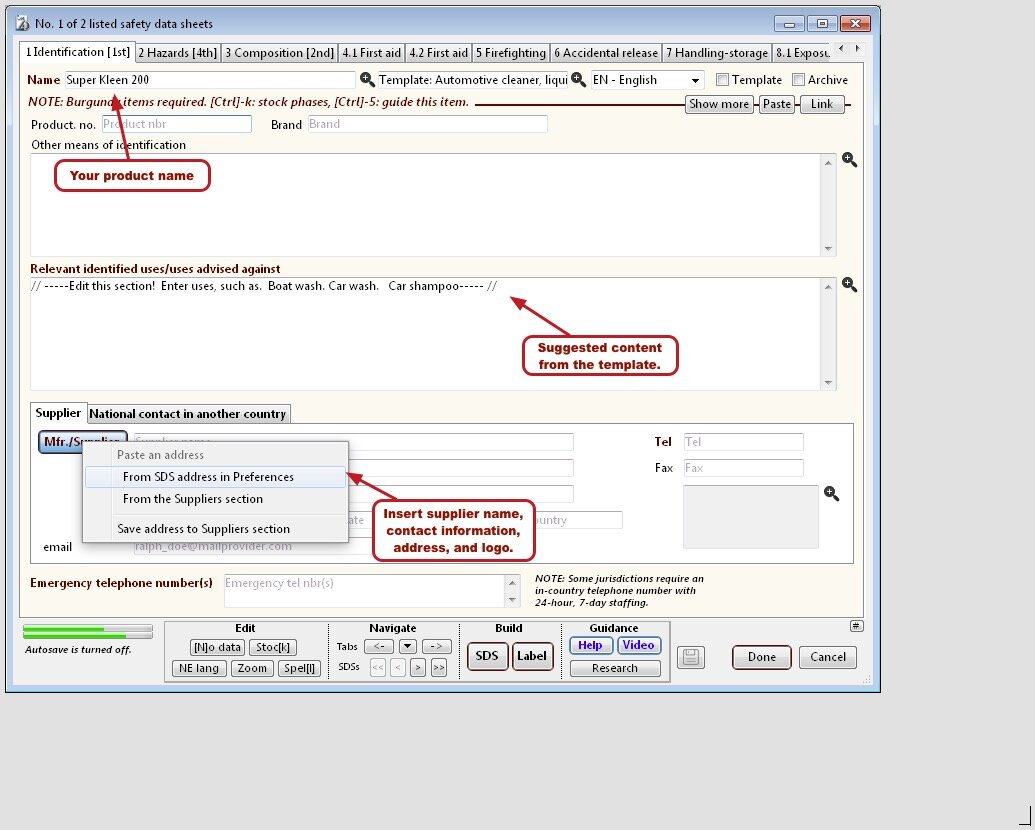
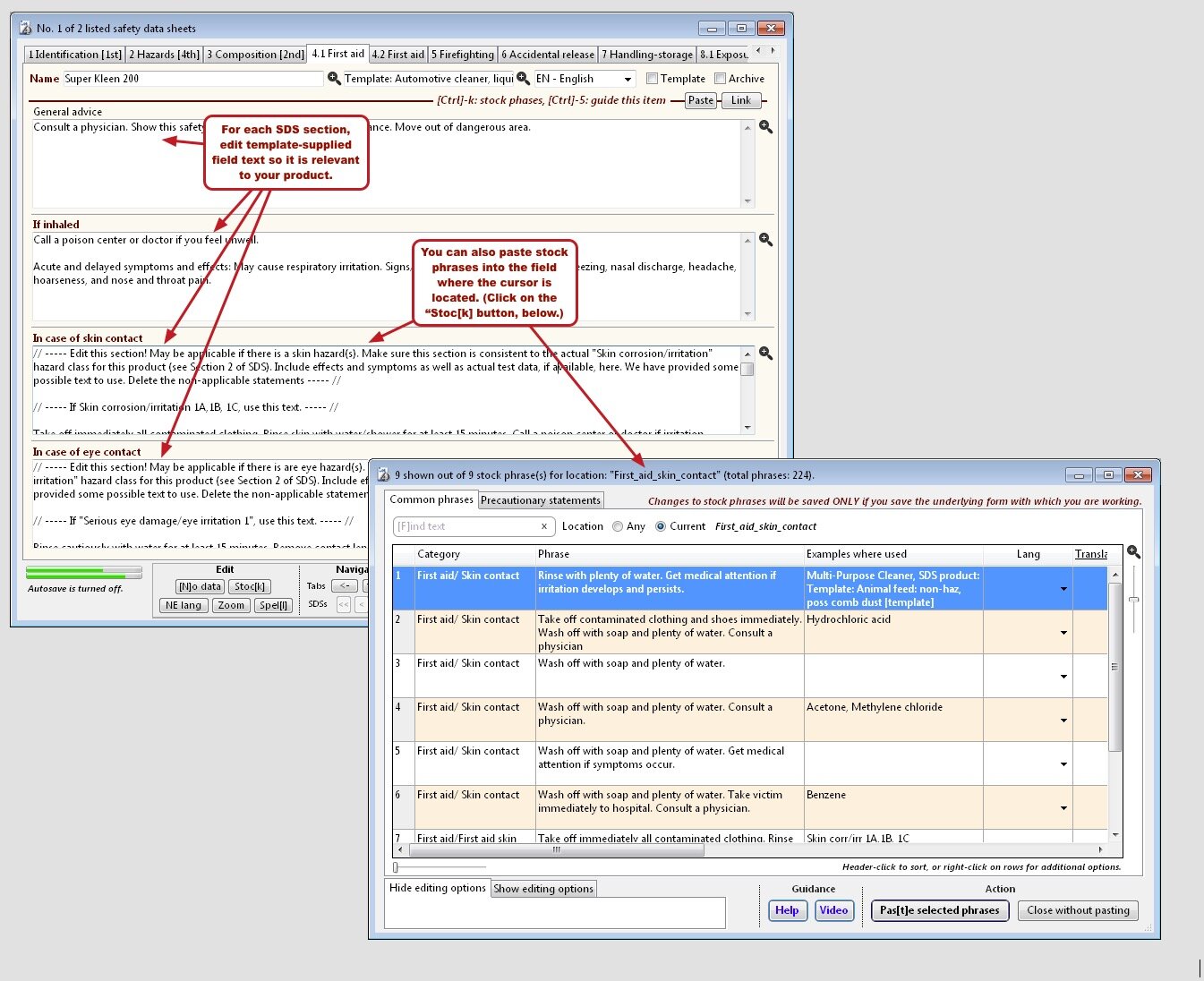
Safety data sheet templates
To get you going quickly, SDScribe™ includes templates for buffers and drug candidates, as well as for products with more generic hazards, like corrosivity and irritation.
Inventory and Production Manager
With the optional Inventory and Production Manager, you can maintain stock levels of ingredients, parts, and finished product; print batch sheets for the production floor and labels for bottles; log batch size, QC analyses, photos and notes; calculate costs; and view calculated VOCs.