FDA labels
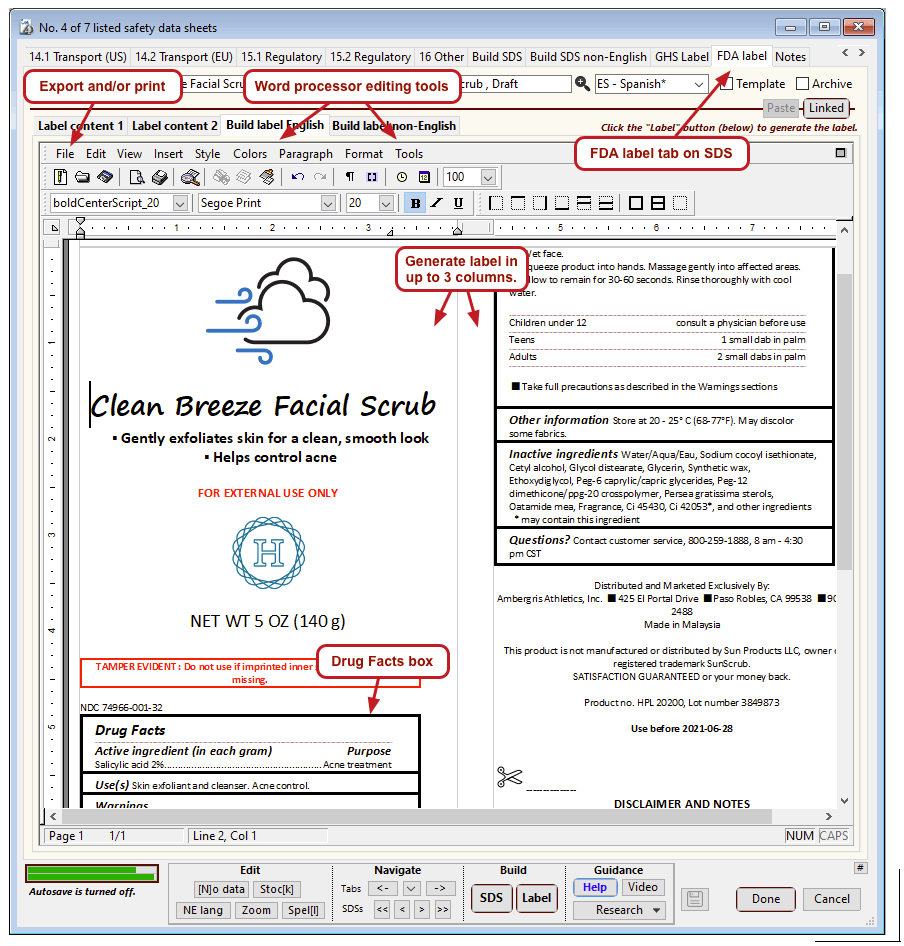
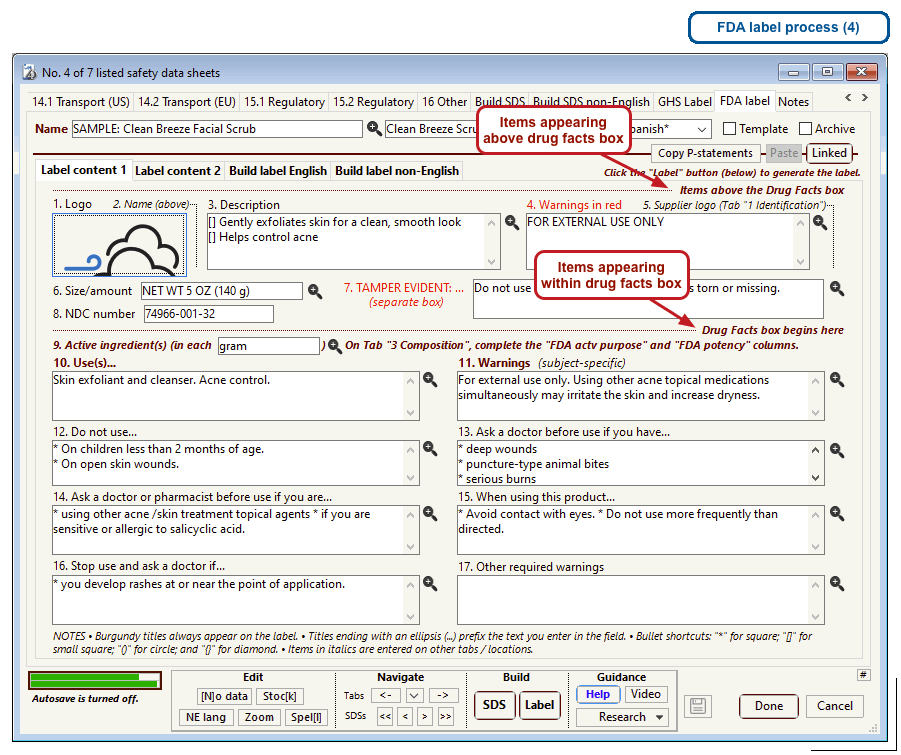
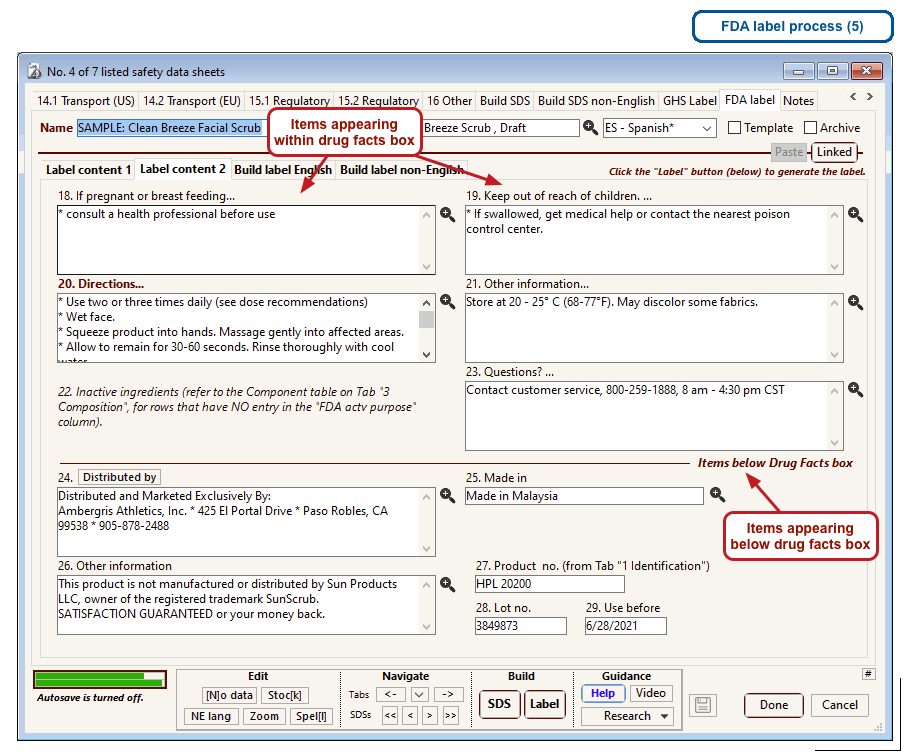
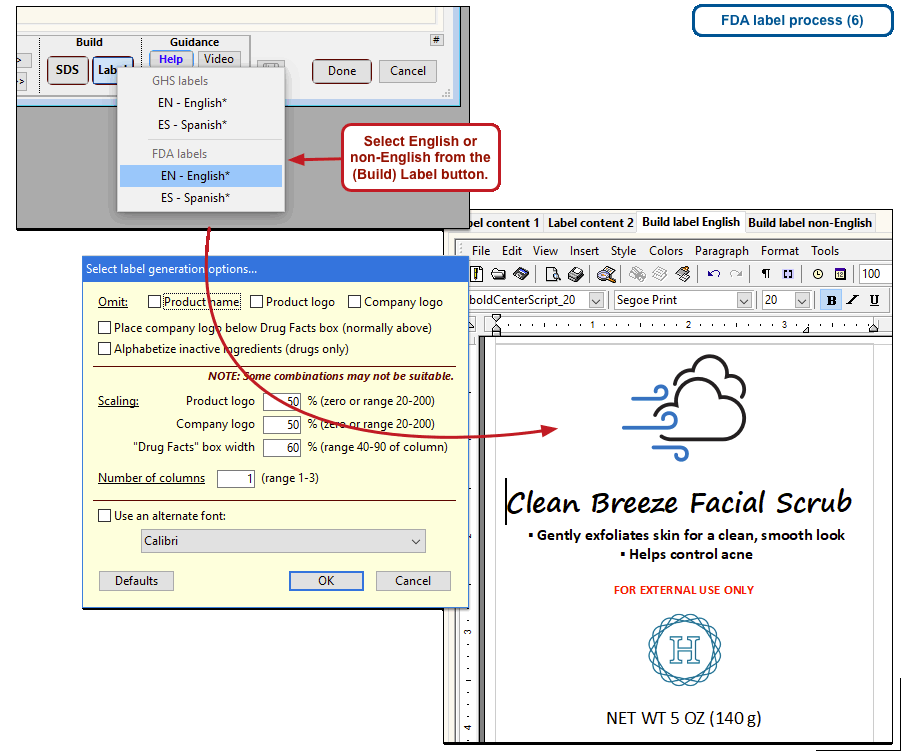
The US Food and Drug Administration (FDA) specifies a format for labels on over-the-counter drugs and other consumer products that have active ingredients, such as sunscreens and antiperspirants. Central to this format is a “Drug Facts” box that includes instructions on product usage and dose, precautions, a list of ingredients, and contact information for questions.
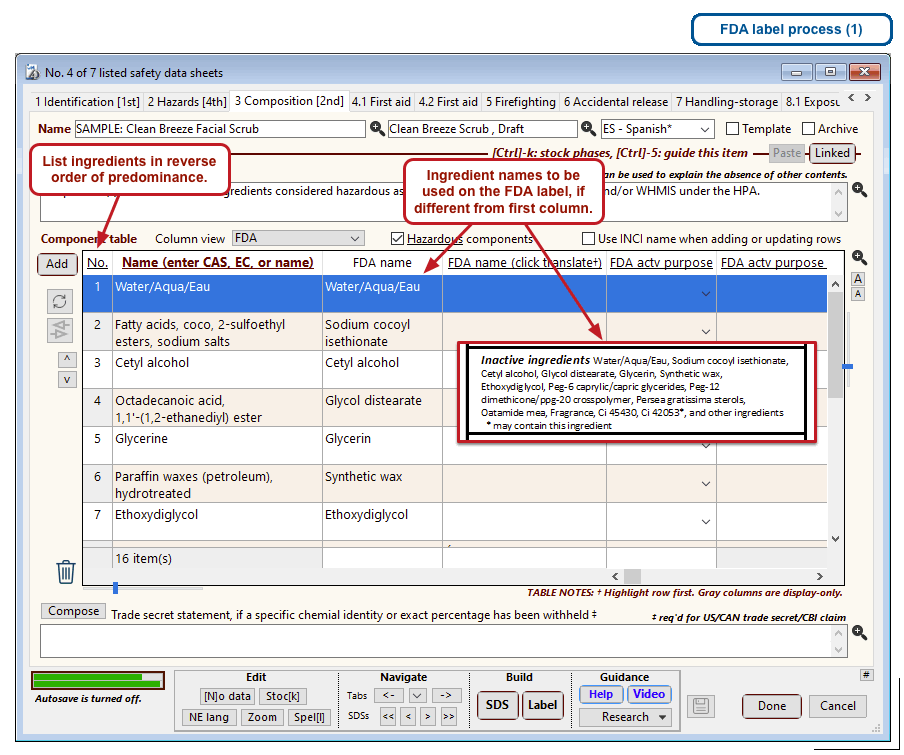
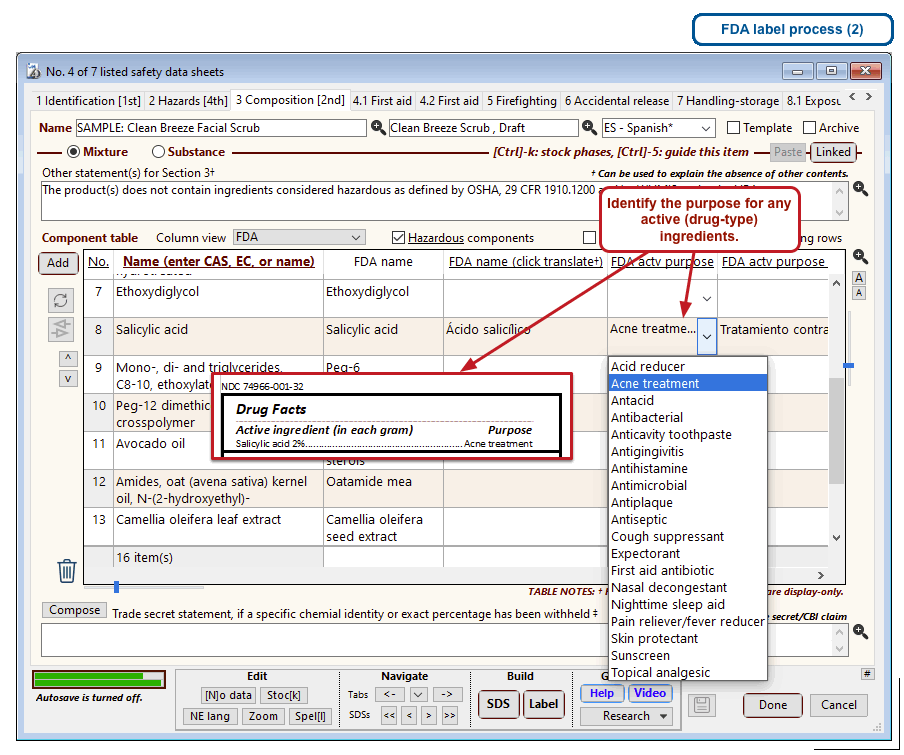
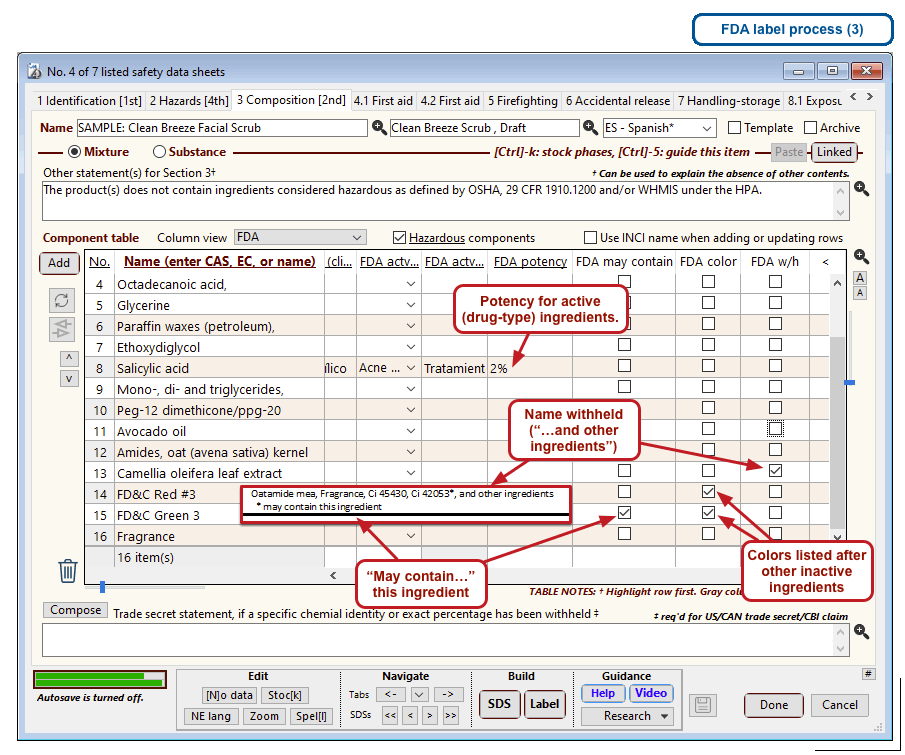
The optional FDA labels feature of SDScribe™ leverages the Safety Data Sheet component list to separate active ingredients from cosmetic ones, colorants, fragrances, and other inactive ingredients. An “FDA label” tab enables you add the other details, and then to generate fully-editable FDA labels. You can customize, print, and/or export them, as you wish.